New light microscopy techniques with esoteric names such as MERFISH, STORM, ORCA, OPS and SIM emerge on what feels like a monthly basis. Each one has its own technical refinements and application niche. It is perhaps surprising then, to find that just two multiline laser illuminators, Lumencor’s ZIVA and CELESTA Light Engines, can fulfill the lighting requirements of all of these techniques. Dr. Bogdan Bintu, Assistant Professor of Bioengineering at the University of California San Diego (UCSD) is well-placed to make this assessment, both from his current research in applying MERFISH to study neurogenesis in aged mammalian brains (Figure 1) and his previous experience in Dr. Xiaowei Zhuang’s laboratory at Harvard University.
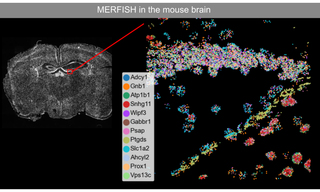
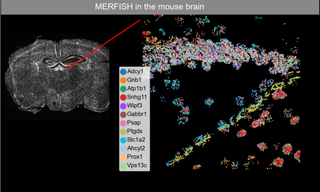
Figure 1. MERFISH imaging of 12 RNA transcripts in mouse brain tissue.
By definition, all light microscopy techniques require an illumination source. In the case of modern “alphabet soup” microscopy techniques designed to provide high-resolution spatial mapping of multiple RNA, DNA or protein species over large fields of view, that source is usually an array of two or more lasers with discrete wavelength outputs. The temporal and spatial coordination of the multiple laser outputs is critical to the end result in all cases. At the technique development stage, individual lasers are typically assembled on an optics breadboard with the flexibility in downstream optical alignment and modulation capabilities required to optimize performance. However, once those optimum conditions have been identified and attained, this paradigm does not facilitate widespread adoption of new techniques beyond a few laboratories with the requisite specialized optical engineering skills. The purpose of the ZIVA and CELESTA Light Engines is to facilitate access to novel imaging modalities for investigators whose primary focus is translational research rather than optical engineering. The first design objective of the ZIVA and CELESTA Light Engine products is to generate light outputs that are variable in terms of wavelength but internally consistent in spatial and temporal characteristics from one wavelength to another. The ZIVA and CELESTA are primarily differentiated by their spatial output characteristics (Table 1). The second design objective is to incorporate the performance characteristics outlined above in a compact, integrated structure (Figure 2) providing the robust, stable and maintenance-free illumination necessary to transform a novel technique into a routine data acquisition exercise that may proliferate to a host of end-users.
“Ideal laser illumination for MERFISH, STORM, PALM and PAINT“ -Bogdan Bintu, Ph.D.
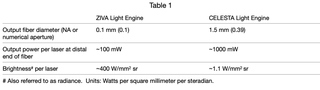

Figure 2. CELESTA Light Engine, showing the overall dimensions and light output port. The ZIVA Light Engine has the same compact dimensions.
STORM (stochastic optical reconstruction microscopy), originally developed in Xiaowei Zhuang’s laboratory, is one of several super-resolution microscopy techniques enabling resolution of objects below the diffraction limit of 200 nm. To achieve this, high irradiance illumination is required to drive stochastic activation of a subset of fluorescent molecules in order to spatially distinguish them from their transiently dark neighbors. At UCSD, Bogdan Bintu and co-workers have implemented STORM imaging using a CELESTA Light Engine coupled via a 400 μm square, 0.22 NA, multimode optical fiber to a simple 3-lens illumination system (Figure 3) allowing for imaging fields of view of up to 100 μm x 100 μm. The images in Figure 4 demonstrate that although the technical nuances of super-resolution microscopy may not be self-evident, the new insights offered to investigators certainly are. Professor Bintu and collaborators recently developed an image processing algorithm (A-Pod) that allows feature extraction from a few, rather than many thousands, of STORM image frames [1].
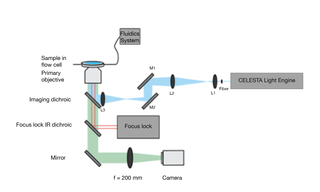
Figure 3. STORM imaging setup with 400 μm fiber output from CELESTA Light Engine and 3-lens optical system
(L3 f = 100 mm, L2 f = 150 mm, L1 f = 8 mm).
Like STORM, MERFISH originated in Xiaowei Zhuang’s Harvard laboratory. MERFISH (multiplexed error-robust fluorescence in situ hybridization) is a massively multiplexed single-molecule imaging technology capable of simultaneously measuring the copy number and spatial distribution of hundreds to thousands of target RNA transcripts in single cells [2]. In turn, single cell transcriptomic characterization allows in situ identification and spatial mapping of cell types in complex tissues (Figure 1). MERFISH imaging requires a high intensity, spatially uniform illumination field matched to the dimensions of typical sCMOS camera sensors (~200 mm2). To meet this requirement, a specialized critical epilluminator coupled to a CELESTA Light Engine via a 400 μm square multimode optical fiber is installed in the microscope, providing 1,000–10,000 mW/mm2 illumination at the sample plane. In typical MERFISH applications, the 477 nm, 637 nm and 748 nm laser outputs of the CELESTA Light Engine are used for iterative identification of DNA probes hybridized to RNA transcripts of interest. 405 nm and 545 nm outputs are used for visualization of DAPI-stained nuclei and microscopic fiducial markers for spatial registration respectively.
Only with bright, stable illumination which embodies spatial and temporal robustness can the capabilities and impact of the newest light microscopy techniques be realized. Pushing past traditional imaging hardware limitations and offering highly multiplexed mapping capabilities required for spatial resolution techniques demands not just more light but well-behaved light. Such turnkey laser Light Engines are a critical component and offer a valuable assist in pushing past historical diffraction limitations to enable such groundbreaking imaging techniques.
“Lumencor’s customization, quick delivery and ability to replicate helps expand our labs capabilities with ease.“
-Bogdan Bintu, Ph.D.
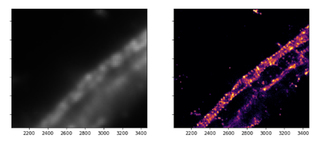
Figure 4. Actin, spectrin and associated molecules form a membrane-associated periodic skeleton (MPS) that plays an important role in the regulation of neuronal function and dysfunction. Its existence was largely unknown prior to the advent of super-resolution microscopy techniques. Diffraction-limited (left) and STORM (right) images (50 μm x 50 μm) of axonal MPS illustrate the limitations and advantages of the resolution enhancements.
- Apr 23, 2024