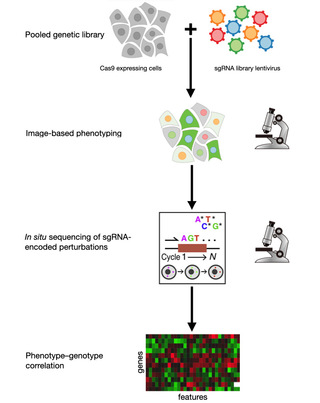
Related Resources
Insights
Case Study: Massively Multiplexed Single-Molecule Imaging Using Lumencor’s CELESTA Light Engine
Insights
Case Study: 3D High-Content Imaging with the CELESTA Light Engine
Lumencorps
More CELESTA and ZIVA Lasers for More Applications
Insights
Epi-illuminators for CELESTA Light Engines in Super-Resolution Imaging
Insights