The human induced Pluripotent Stem Cells (hiPSC) technique was first introduced in 2007 by Dr. Shinya Yamanaka’s research team; a discovery for which he was awarded the 2012 Nobel Prize in Physiology or Medicine [1]. This method is popularized and widely used in biomedical research, with applications ranging from disease modeling to personalized medicine. The De Vrij Lab in the Department of Psychiatry at the Erasmus Medical Center (Erasmus MC), Rotterdam, utilizes hiPSCs to generate living human brain cells in efforts to uncover mechanisms of neurodevelopment and differentiation. This approach allows them to mimic brain development in a micro dish or plate, and study the underlying biological mechanisms of psychiatric and neurodevelopmental disorders [2].
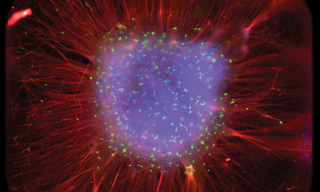
In the increasingly diverse landscape of hiPSC-derived models of brain development, the De Vrij group recently presented a novel method for generating three-dimensional cortical layered organoids [3]. This technique produces highly reproducible and topographically standardized structures in 384-well plates. The resulting self-organized organoids revealed a radial cortical structure with typical dimensions of 3 × 3 × 0.2 mm. Using fluorescence-based calcium imaging, a plethora of brain cell types can be visualized within the organoids, including multiple neuronal subtypes, astrocytes, and oligodendrocyte lineage cells. The spatial organization of the organoids follows an inside-out pattern, where radially deep-layer neurons develop before shallow-layer neurons, thereby mimicking in vivo cortical development. Further, the organoids exhibit robust neuronal activity, indicating the formation of a functional neural network.
Insights gained from organoid fluorescence imaging are crucial for high-throughput drug discovery and neurotoxicological screening applications. To facilitate the rapid and consistent imaging of brain cells and organoids in the De Vrij Lab, Lumencor customized a TARGA Imager. The TARGA Imaging platform recognizes that neuroscience is one among numerous fields requiring a triumvirate of imaging performance traits: fast imaging speed, large field-of-view, and sensitivity to enable visualization and measurement of intricate and multifaceted neuronal structures. Unlike conventional, more time-consuming widefield fluorescence and confocal scanners, which acquire multiple images serially and then require tiling to capture the entire 3 × 3 mm sq well area, the TARGA images the entire large fields-of-view, whole well areas, instantaneously (Figure 1). This capability facilitates the study of dynamic properties of organoids, such as their short-term development and disease progression. It also eliminates the need for laborious and imprecise image-stitching, ensuring consistent and undistorted visualization. TARGA can reduce experimental time by an order of magnitude, versus traditional laser scanning confocal studies, with no loss of information. Given its capacity to capture high speed data at faster than video rates, the TARGA Imager supports up to 1 TB of memory storage to provide a smooth workflow and efficient data transfer. Further, TARGA’s fast multicolor illumination system, enables the instantaneous imaging of distinct brain cells labeled with an array of fluorescent markers throughout the UV-VIS-nIR spectrum.
Like all Lumencor products, the TARGA Imager platform represents a family of products. Should your imaging demand unique spectral, spatial and/or temporal control, outside the constraints of traditional fluorescence microscopy techniques, TARGA can be tailored to meet your customized specification. TARGA is pushing the boundaries of bright-field and fluorescence imaging with hardware built around the high-performance, solid-state lighting upon which Lumencor has built her reputation. How can Lumencor help to enable your best imaging experiments?
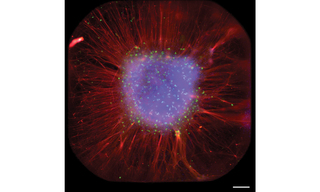
Figure 1. Full well acquired by TARGA showing radial organization of organoid. Scale bar is 0.2 mm. Image by Sakshi Bansal